X2 Y2 Orbital
The importance of the orbital degree of freedom is pointed out as well as the charge and spin ones.

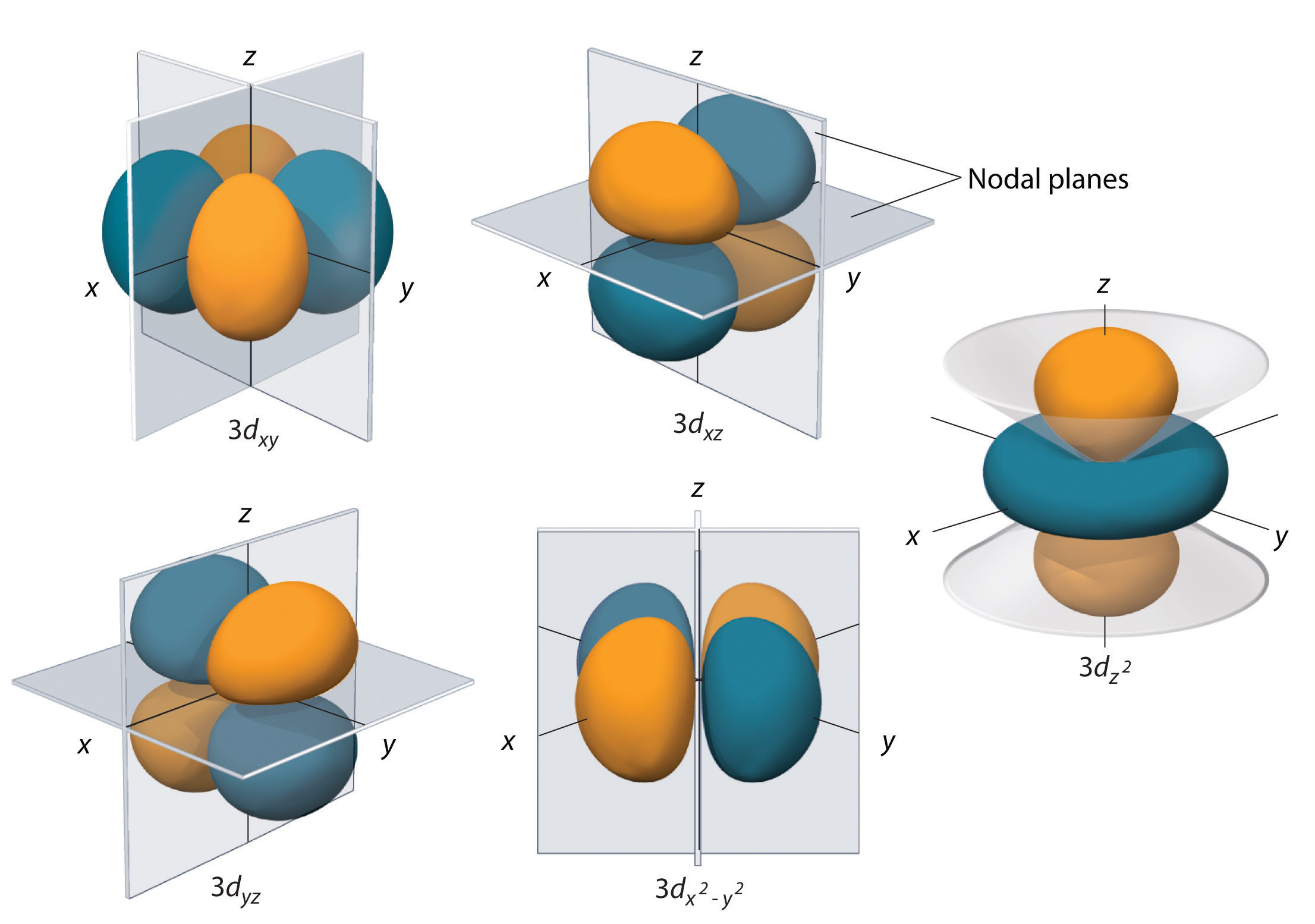
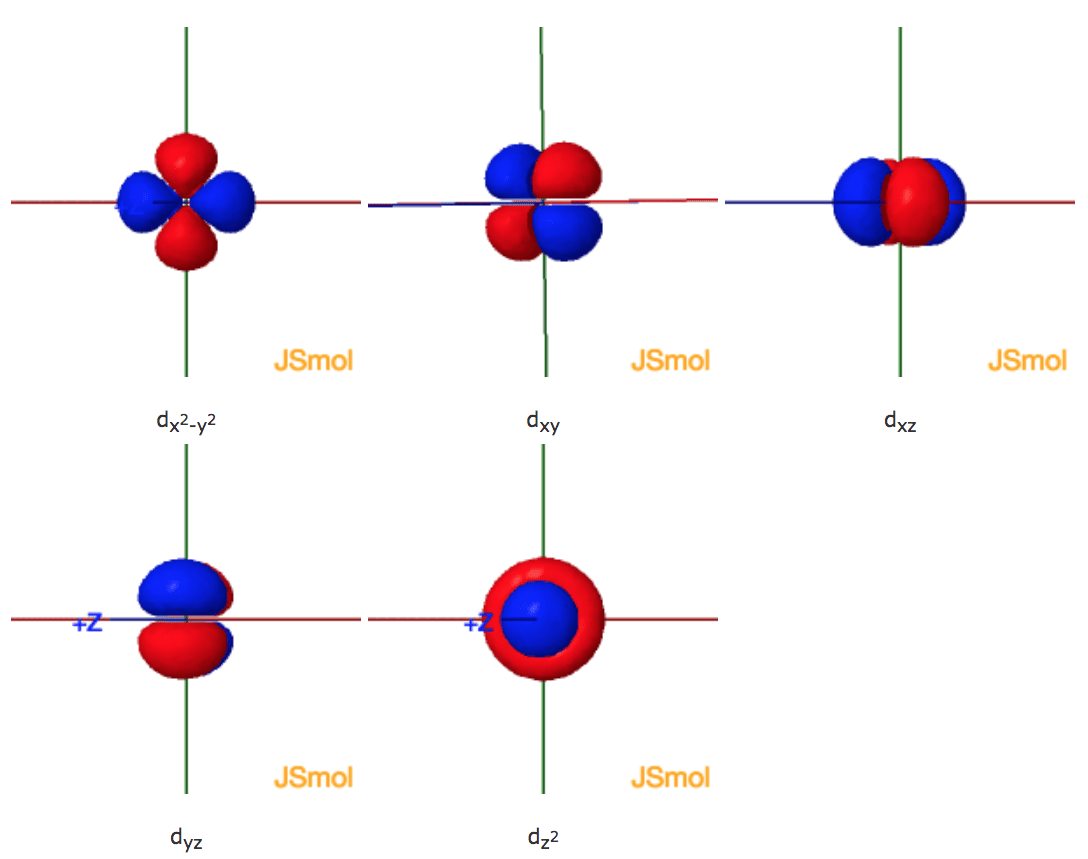
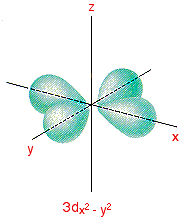
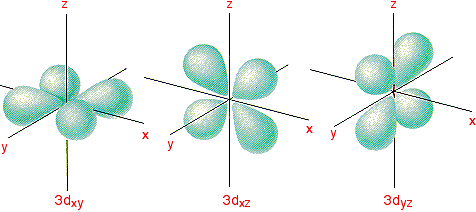
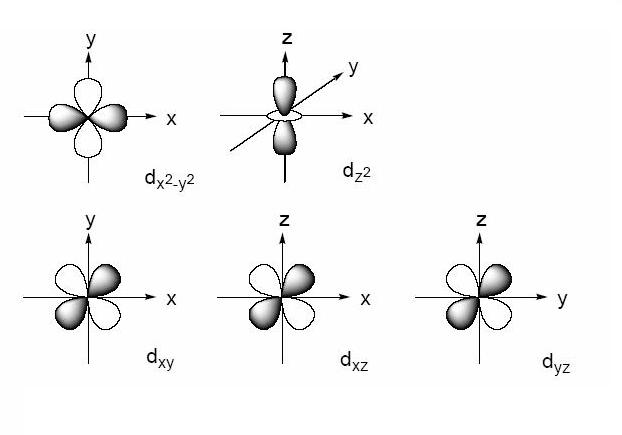
X2 y2 orbital. The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. B.The value of ℓ would increase by 2. Thus d orbital corresponds to 4 double dumb-belled shapes (d xy, d yz, d zx, d x 2 y 2) with the atomic nucleus at its centre and one dumb belled with dough nut shaped (d z 2).
However, two of the electrons in the d x 2 − y 2 and d z 2 orbitals would occupy the d z 2 orbital, whereas only one would be in the higher energy d x 2 − y 2 orbital. Hence d orbitals have five orientations in space. D zx, d x 2-y 2 and d z 2;.
For example, 3d xy, 3d yz, 3d zx, 3d x 2-y 2 and 3d z 2. 2 refers to the next energy level further out, and so on. School University of Ontario Institute of Technology;.
C.The radial probability function would include two more nodes. If there is only crystal field splitting and no Jahn-Teller distortion (In a. The symbols used contained in right here are:.
Click here👆to get an answer to your question ️ The species in which (n - 1)dx^2 - y^2 orbital take part in hybridization?. A fourth d orbital has lobes lying along the x and y axes;. The sp, sp 2 and sp 3 Hybrid Orbitals.
13a) of the central ion has the same symmetry as the σ group orbitals of the ligand system shown in Fig. The value of l would increase by 2. Dx2, dxy, dxz, dzx, d(x2-y2) - Orbital Model Sets - Kit of 1:.
This is the \(3d_{x^2−y^2}\) orbital. This question has multiple correct options. We have investigated the orbital state in La2-2xSr1+2xMn2O7 by.
S p 3 d 3. 13 the d x 2 − y 2 orbital (Fig. Industrial & Scientific Skip to main content Try Prime.
Likewise d z2 (along z) does not contact the negative charges. Let's proceed to look at the s - Orbital. Dxy , dyz , dxz , dx2-y2 and dz2 All the d-orbitals have the same shape , Double Dumbbell.
In this simplecase,therecanbeanorbital-selectiveMotttransi-tion(OSMT)ifthedensityofoneorbitalisatn 1 = 117– 19. This is the hard way:. X2−y2 state, with mass enhancement of ∼ 2.4.
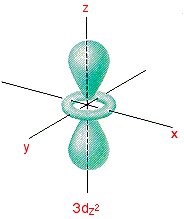
Which of the above fourth shell orbitals is a 4 d x 2. Thus their product is an odd function, and when evaluating the integral of an odd function over a symmetric interval (and doing so over all space is necessarily a symmetric interval. The d z 2 orbital has a somewhat novel configuration, in which two lobes lie on the z axis and are encircled by a doughnut-shaped orbital centred on the z axis.
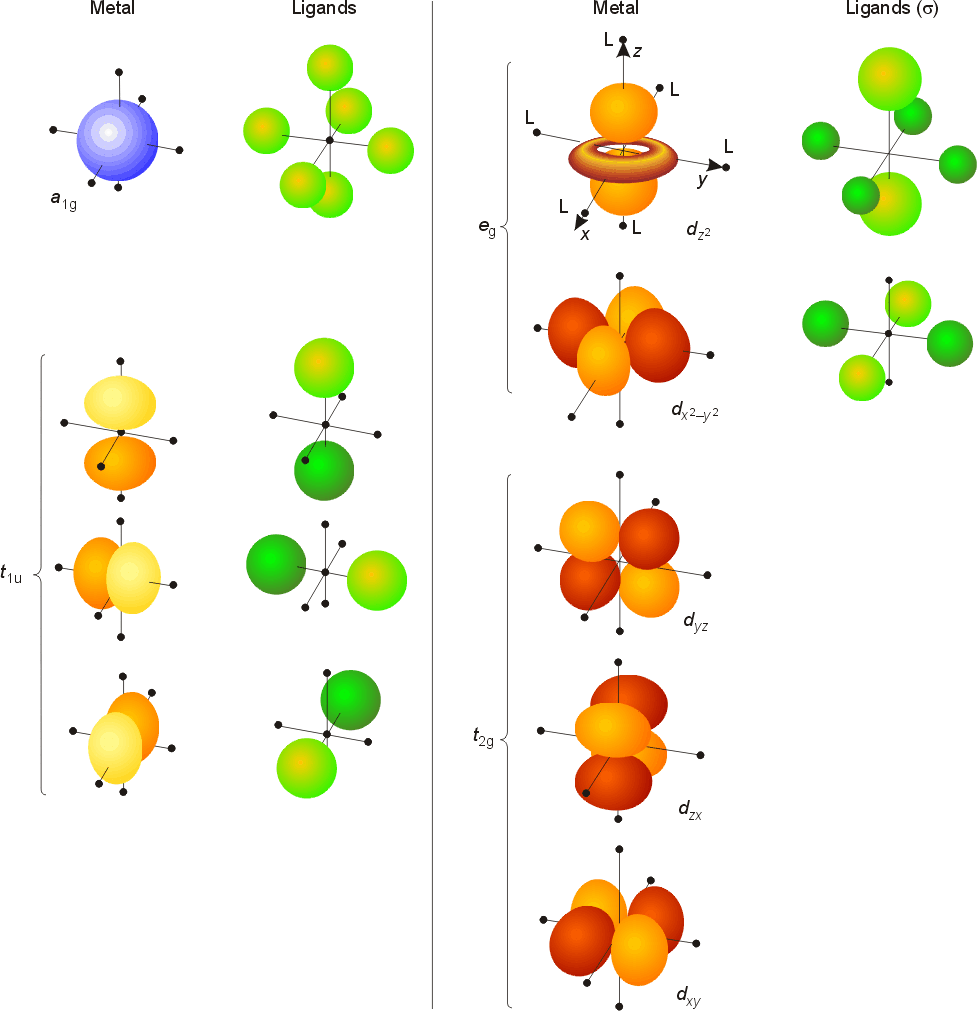
D sub-shell has 5 orbitals:. S p 3 d 2. Chem 59-250 Symmetry of orbitals and functions yz y, R x 1-1-1 1 B 2 xz xy x 2,y 2,z 2 x, R y R z z-1 1-1 1 B 1-1-1 1 1 A 2 1 1 1 1 A 1 σ ’ v (yz) σ v (xz) C 2 E C 2V z y x z y x A p z orbital has the same symmetry as an arrow pointing along the z-axis.
This means that the populations of x2 –y2 and 3z 2 – r 2 orbitals in the e g state play a key role in understanding the transport and magnetic properties of this system. It is quickly suppressed to ∼ 2.1 upon heavy hole-doping. Also, the p +1 and p −1 are not the same shape as the p 0 , since they are pure spherical harmonics.
I dont understand these orbitals and how they would compare to each other. 13c, therefore a combination between these two can be made. This 3D model was originally created with Sketchup 7 and then converted to all other 3D formats.
The p z orbital is the same as the p 0 orbital, but the p x and p y are formed by taking linear combinations of the p +1 and p −1 orbitals (which is why they are listed under the m = ±1 label). Light with helical phase fronts is associated with orbital angular momentum (OAM) states denoted as φ (r, ϕ) = exp (i l ϕ), where ϕ is the angular coordinate and l can take any integer value. What is the probability of occupying 3z2-r2 or x2-y2 orbital for the 7th electron of a d7 configuration metal ion?.
We believe these findings are helpful to understand the unconventional super-conductivity in this system. Related Questions to study. 1 2 = 1, 2 2 = 4, 3 2 = 9.
However, $\ce{d_{x^2-y^2}}$ doesn't change signs under the same transformation and thus is even. A.The contour of the orbital would extend further out along the x and y axes. The Chime plugin (Version 2.0 or higher) is required to view this page.
The only difference between these two orbitals is that the d_(x^2-y^2) lobes are along the axes and the d_(xy) is rotated 45^@ counterclockwise. Solving true or false. Notice that these orbitals are all very similar, in that the majority of the orbital is oriented in a particular direction.
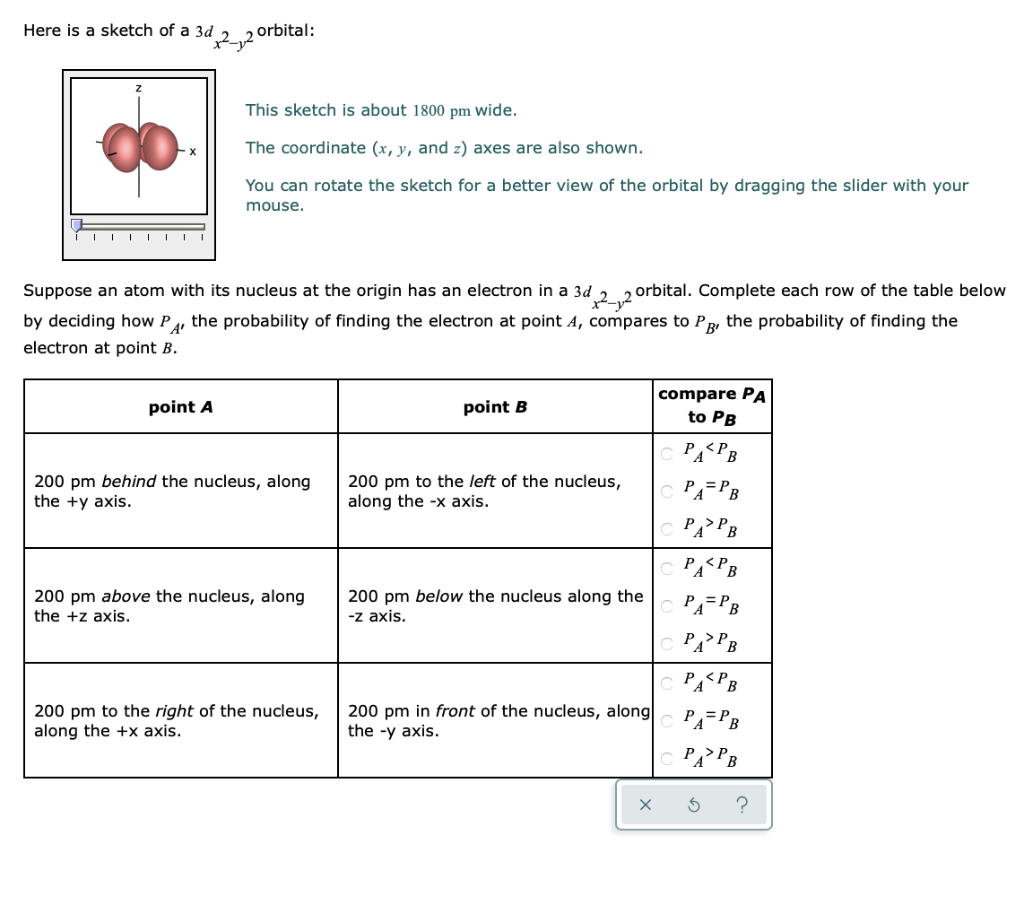
Prev Question Next Question. Here is a sketch of a 3d 2. D x2-y2 d xy d z2 e set (stabilized) d yz t 2 set d xz (destabilized) z x y.
Model with a weak inter-orbital coupling6,7. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. H:Course BackupsChem 103Chem 103 Originals For Brown 10th edOverheadsCh103BrownCh6ov7.wpd Author:.
The things we had to say were true or false were:. How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell?the contour of the orbital would extend further out along the x and y axes.the value of ℓ would increase by 2.the radial probability function would include two more nodes.the orientation of the orbital would be rotated 45∘ along the xy plane.the mℓ value would be the same.drag the. In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties and are.
Dz2 ) ” However , each of the 5 orbitals have different spacial orientations.These spacial orientations are determ. The other orbital can have a generic filling n 2 = x and forms a small Fermi pocket. Which of the above fourth shell orbitals is a 4 d x 2 y 2 orbital A orbital a B.
Most likely, your textbook will include a discussion of the derivation for at least the octahedral case. The spin moment of the Mott localized orbital couples to the itinerant Fermi pocket through a. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape:.
The number denotes the energy level of the electron in the orbital. X-axis = yellow y-axis = green z-axis = blue:. 0606K + pkg - Set of 5 d Orbitals:.
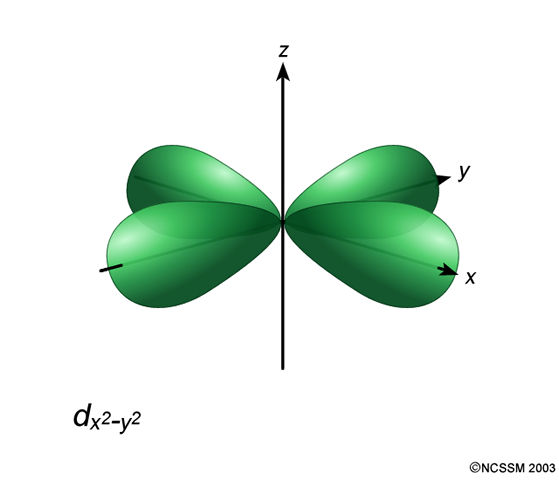
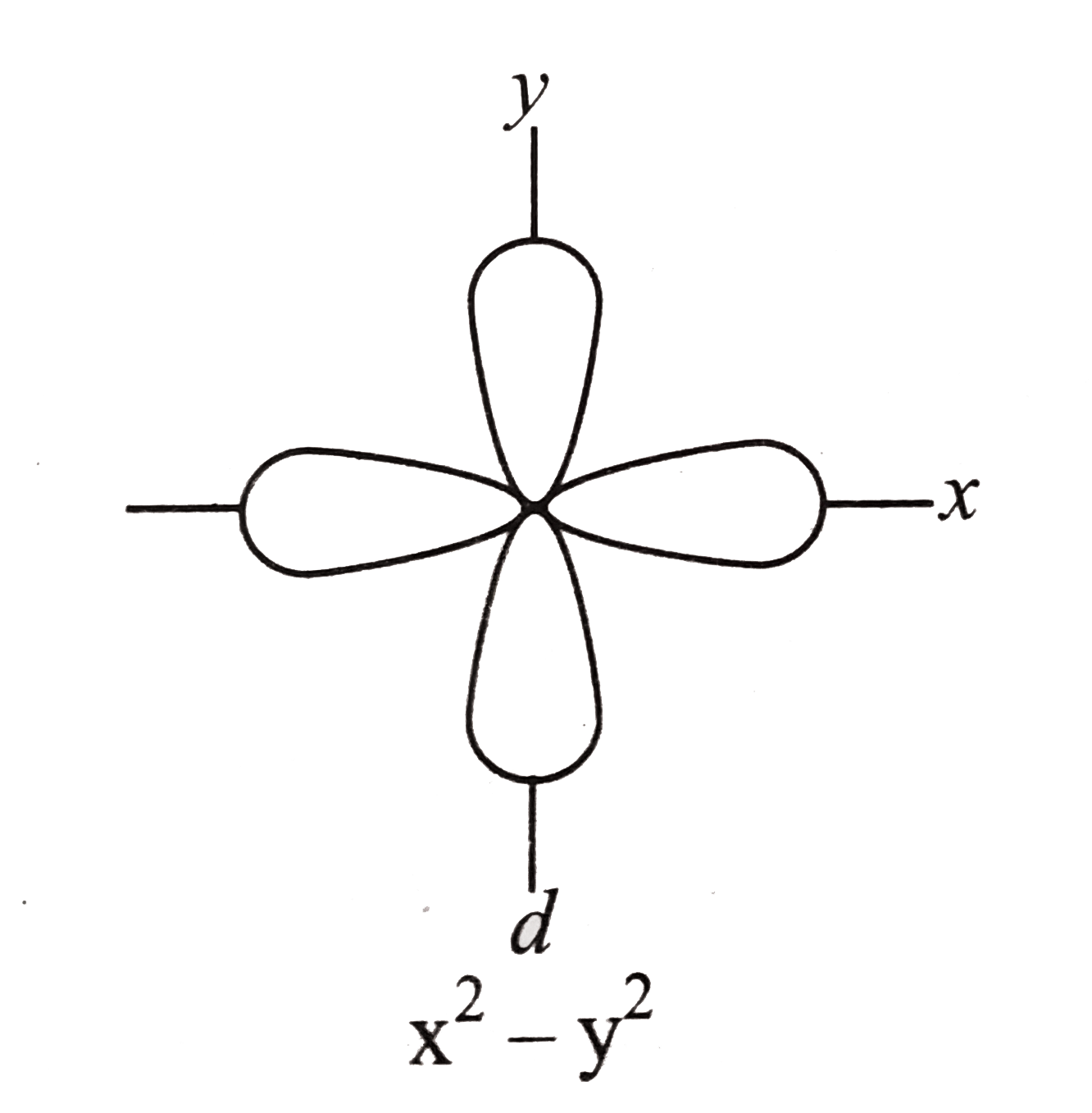
In the d x 2-y 2 orbital, one pair of lobes lies on the x axis and the other lies on the y axis. The doughnut is bisected through its circumference by the plane formed by the x and y axes. For instance, the corre-lated Ni-3d x2−y2 orbital is most relevant for electron pairing, and the change of topology of Fermi surface.
Orbitals are the regions of space in which electrons are most likely to be found. Ratings 92% (13) 12 out of 13 people found this document helpful. For the 3d yz orbital lies in the plane defined by the y and z axes, and between the y and z axes, for the 3d x 2-y 2 orbital lies in the plane defined by the x and y axes, and directly along the x and y axes, and:.
The d z 2 orbital looks very different from the other four:. X The coordinate (x, y, and z) axes are also shown. Even though the d z 2 orbital looks different, it has the same energy as the other four d orbitals.
= 3. approximately e = 2.718 approximately Z = effectual nuclear fee for that orbital in that atom. Orbital will have more contact with the negative charges than the d x2-y2 orbital (no charges point at the d x2-y2 orbital but the d xy orbital is between the axes). The angular wave function for the hydrogen atomic 3d_(x^2-y^2) orbital ((l,m_l) = (2,2)) is given by:.
Robert Carter Created Date:. What Is A Hybrid Orbital?. Stack Exchange network consists of 176 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers.
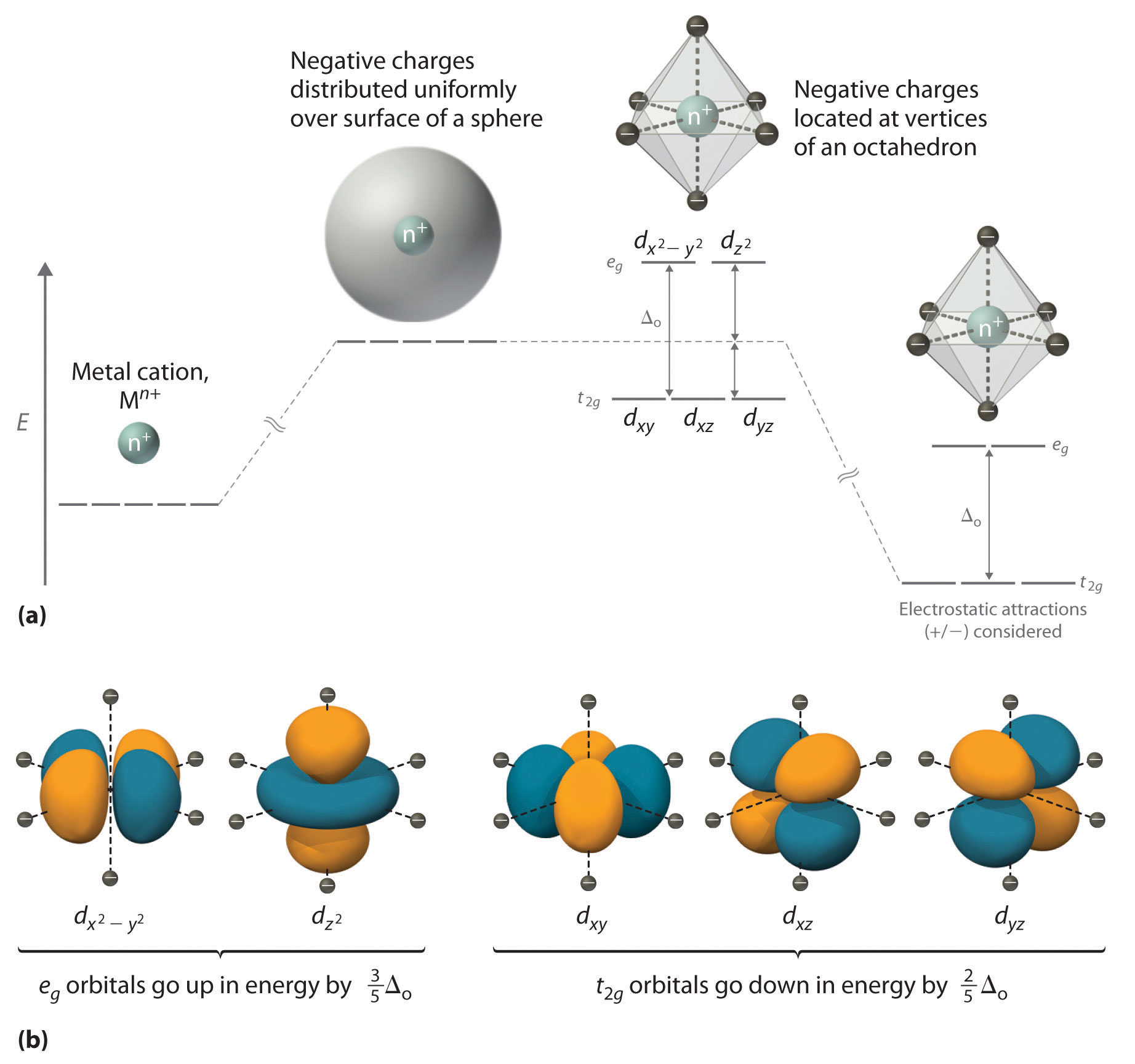
For the 3d z 2 orbital lies directly along the z axis. For d orbital Azimuthal quantum number l = 2 and the magnetic quantum number m = -2, -1, 0, +1, +2. As a result, the energy of this arrangement would be lower than that for the complex having O h symmetry.
The splitting of the two sets of orbitals (e g and t 2g) is not equal. This sketch is about 1800 pm wide. S, p x, p y, d x 2-y 2 The electron density plots below compare the sp, sp 2 , and sp 3 orbitals.
It looks like a \(2p_z\) orbital combined with an additional doughnut of electron probability lying in the xy plane. Expanded View of the 3d x 2-y 2 Orbital. E.The mℓ value would be the same.
The d_(x^2-y^2) has two vertical nodal planes bisecting the x and y axes, and the d_(xy) has an bb(xz) and bb(yz) nodal plane. The average distance between the nucleus and the 2s electron is shorter than the average distance between the nucleus and 3s electron. The px orbital has antinodal regions (or cloud density) located along the x axis.
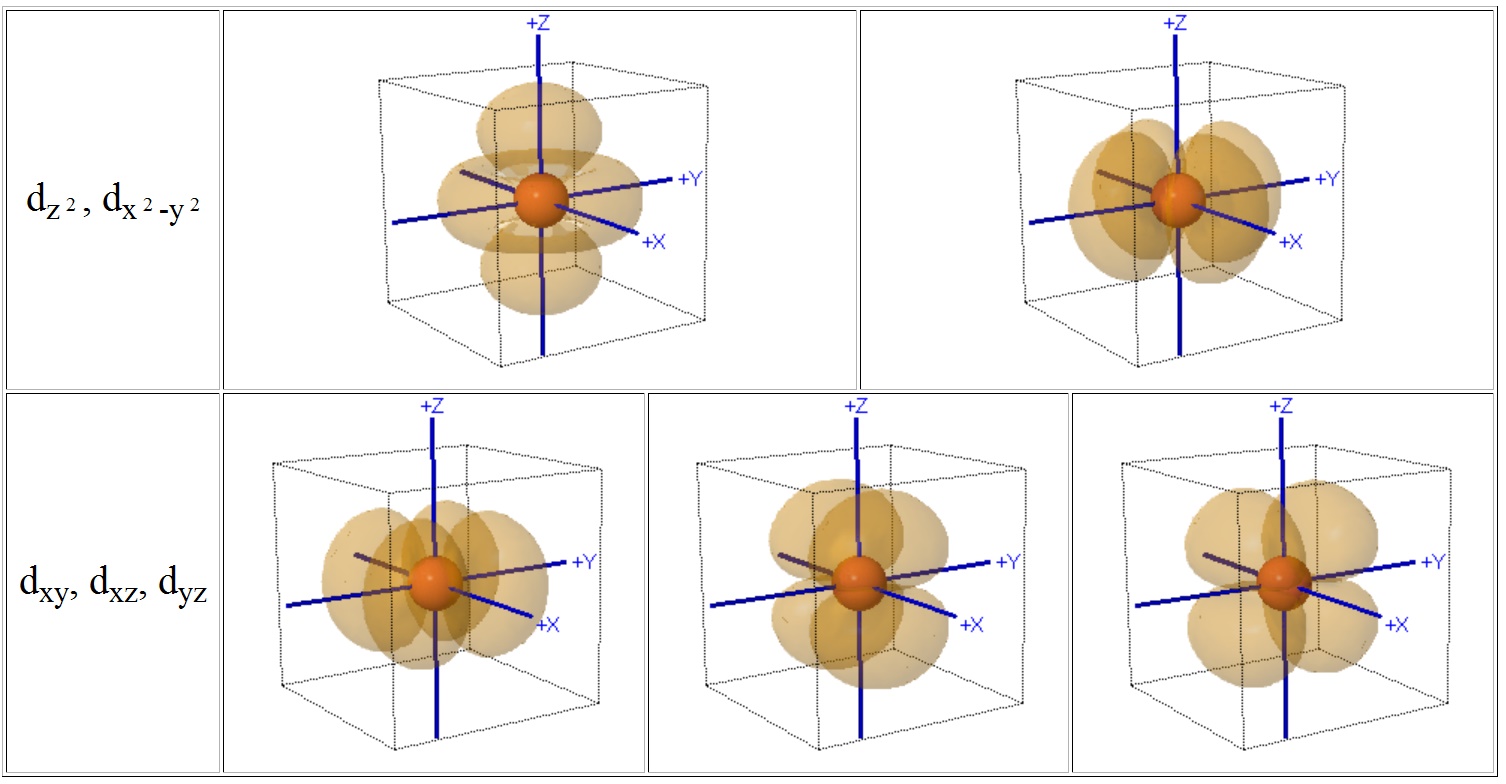
The combination orbitals must have the same symmetry. The d xy , d yz and d zx orbitals have same shape i.e., clover leaf shape but they lie in XY, YZ and ZX- planes respectively.The d z2 orbital is symmetrical about Z-axis and has a dumb - bell shape with a doughnut shaped electron cloud in the centre. There are rigorous mathematical ways to deduce the symmetry labels of d-orbitals (or any orbital, for that matter) under an environment of a certain symmetry, as provided by point group theory, which involve symmetry tables and so on.
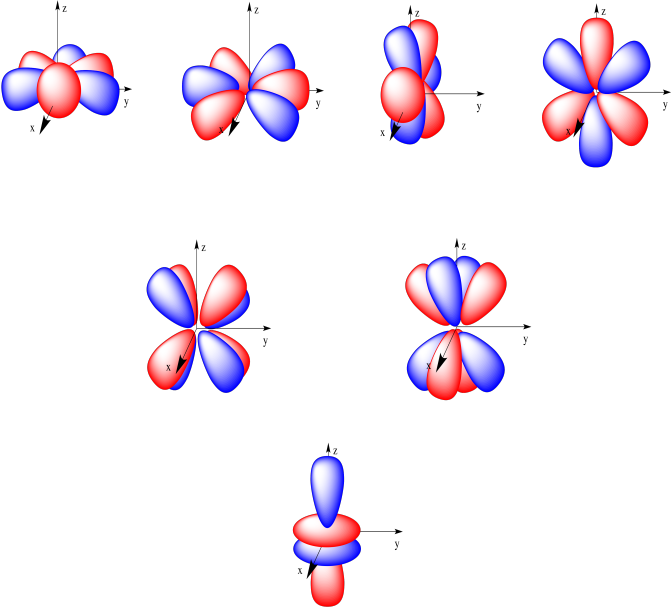
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. X y z 3d Orbitals n = 3, l = 2, ml = +2, +1, 0, -1, -2 3dxy 3dxz 3dyz 3dx2-y2 3dz2 L The 3dxy, 3dxz, and 3dyz orbitals’ lobes are between the axes in their names. = 2Zr/n the place n is the critical quantum quantity (3 for the three-D orbitals) (Schrödinger wave equations) Radial wave function, R3d.
Y_(2)^(2)(theta,phi) = 1/4 sqrt(15/(2pi))sin^2thetae^(2iphi) The nodes occur when this function is equal to zero. The radial probability function would include two more nodes. Look at the s - Orbital?.
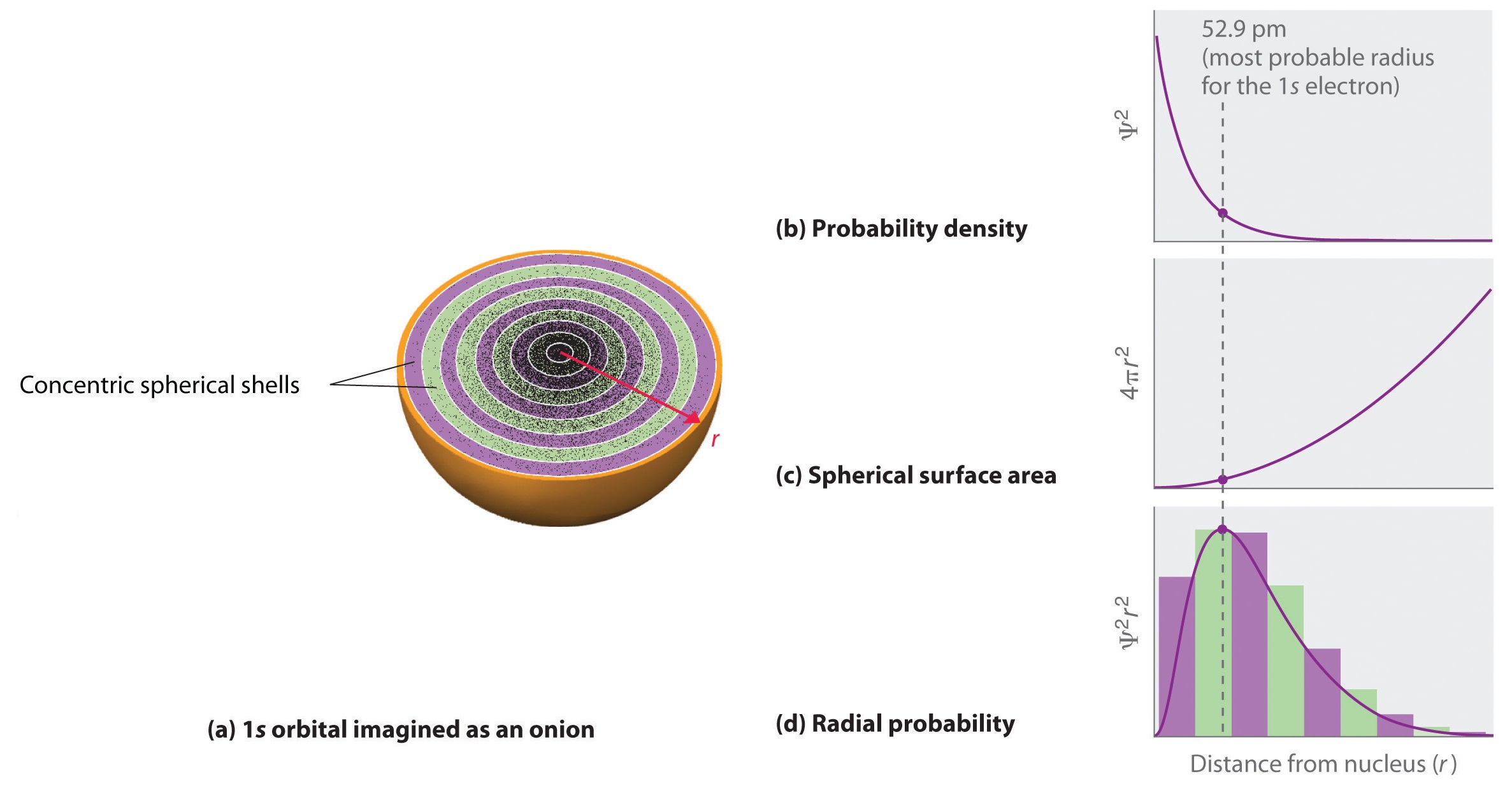
R = radius expressed in atomic gadgets (a million Bohr radius = fifty two.9 pm) ?. This is evident if you look at the shape/diagram of the orbital. Each orbital is denoted by a number and a letter.
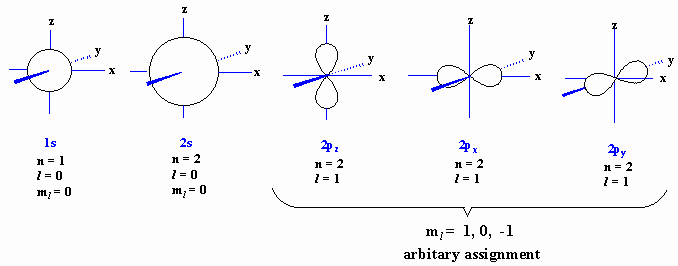
L The 3dx2-y2 orbital’s lobes are on the x and y axes. The fifth 3d orbital, called the \(3d_{z^2}\) orbital, has a unique shape:. Thus 1 refers to the energy level closest to the nucleus;.
The number of orbitals in a shell is the square of the principal quantum number:. Sx_, y_, z_ := Exp-(x^2 + y^2 + z^2) This is NOT the function given by Quantum Mechanics, this were but the function chosen here is more convenient if calculations should be made and is well appropriate for further discussions. D orbital has two.
The contour of the orbital would extend further out along the x and y axes. You can rotate the sketch for a better view of the orbital by dragging the slider with your mouse. For example, in Fig.
The dx2 - y2 orbital has charge density located along the x and y orbitals. It looks like a \(2p_z\) orbital combined with an additional doughnut of electron probability lying in the xy plane. A fourth d orbital has lobes lying along the x and y axes;.
D.The orientation of the orbital would be rotated 45∘ along the xy plane. Suppose an atom with its nucleus at the origin has an electron in a 3d 2 2 orbital. The lobes of the d x 2 - y 2 orbital also lie in the xy plane, but the lobes lie along the x and y axes.
It is x^2 – y^2. Course Title CHEM 1800U;. It has two lobes along the z axis and a "doughnut" in the xy plane.
This is the \(3d_{x^2−y^2}\) orbital. Native format is .skp 3dsmax scene is 3ds Max 16 version, rendered with Vray 3.00 d x2y2 orbital superimosed on an octahedral model. But what does that mean:.
S p 3 d 2 involves d z 2 and d x 2 y 2 whereas s p 3 d 3 uses d x 2 y 2, d z 2 and d x y. D x 2 − y 2 orbital is involved in which the following hybridization:. More than 1correct answer!.
Woerdman, “ Orbital angular momentum of light and the transformation of Laguerre-Gaussian laser modes,” Phys. For math, science, nutrition, history. The orientation of the orbital would be rotated 45 degrees along the xy plane.

Lecture 17 Molecular Orbital Theory 1 Molecular Orbitals Of Ah X X 3 4 6 Mo Diagrams Can Be Used On A Qualitative Basis To Understand The Shape Ppt Download
A Energy Levels
Solved Here Is A Sketch Of A 3d 2 2 Orbital This Sketch Chegg Com
X2 Y2 Orbital のギャラリー

Total Dos In A Afm Magnetic And X 2 Z 2 Y 2 Z 2 Orbital Orderings Download Scientific Diagram
Solved Generate D Orbital Splitting Diagrams For The Foll Chegg Com
2 8 Bonding In Octahedral Complex Ions Crystal Field Theory Chemistry Libretexts
Crystal Field Theory Cft

Orbit Subshell Orbital Electron

Quantum Numbers
Ch 1 Electrons And Orbitals
6 6 3d Representation Of Orbitals Chemistry Libretexts
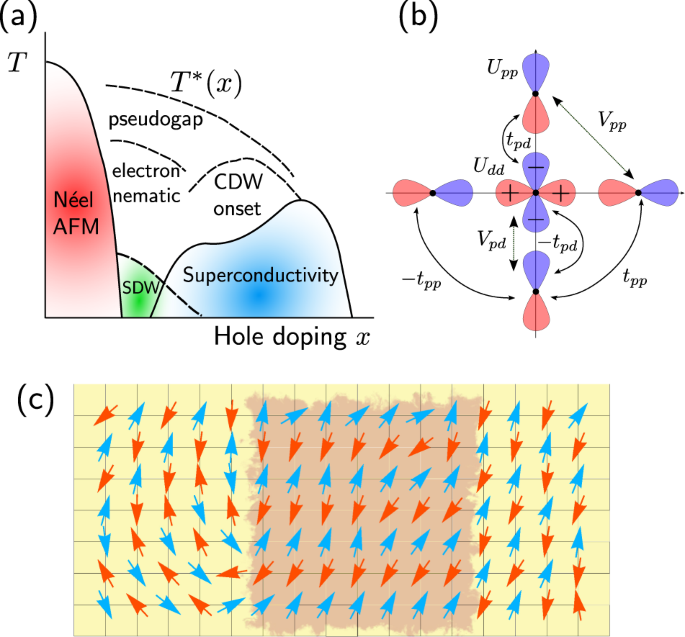
Enhanced Nematic Fluctuations Near An Antiferromagnetic Mott Insulator And Possible Application To High T C Cuprates Npj Quantum Materials

Which Group Of D Orbitals Has Greater Energy Quora

Figure 4 Orbital Ordering Scenarios For Jahn Teller Active 3 I D I Sup 9 Sup And 4 I D I Sup 9 Sup Layered Perovskites

Bonding In Co Ordination Compounds Study Material For Iit Jee Askiitians

Orbitals Definition Types Orbital Shapes Quantum Numbers

Molecular Nitrogen And Related Diatomic Molecules
D Metal Complexes

The D X 2 Y 2 Orbitals Of Fermions On Copper Ions And Px And Py Download Scientific Diagram

Use The Molecular Orbital Energy Level Diagram To Show That N 2
Q Tbn 3aand9gcsn9uq1db Ygak2ljk9orermnbntstwqgpmxsqtrsodds2ezru Usqp Cau

What Is The Difference Between Dx2 Y2and Dz2 Orbitals Quora
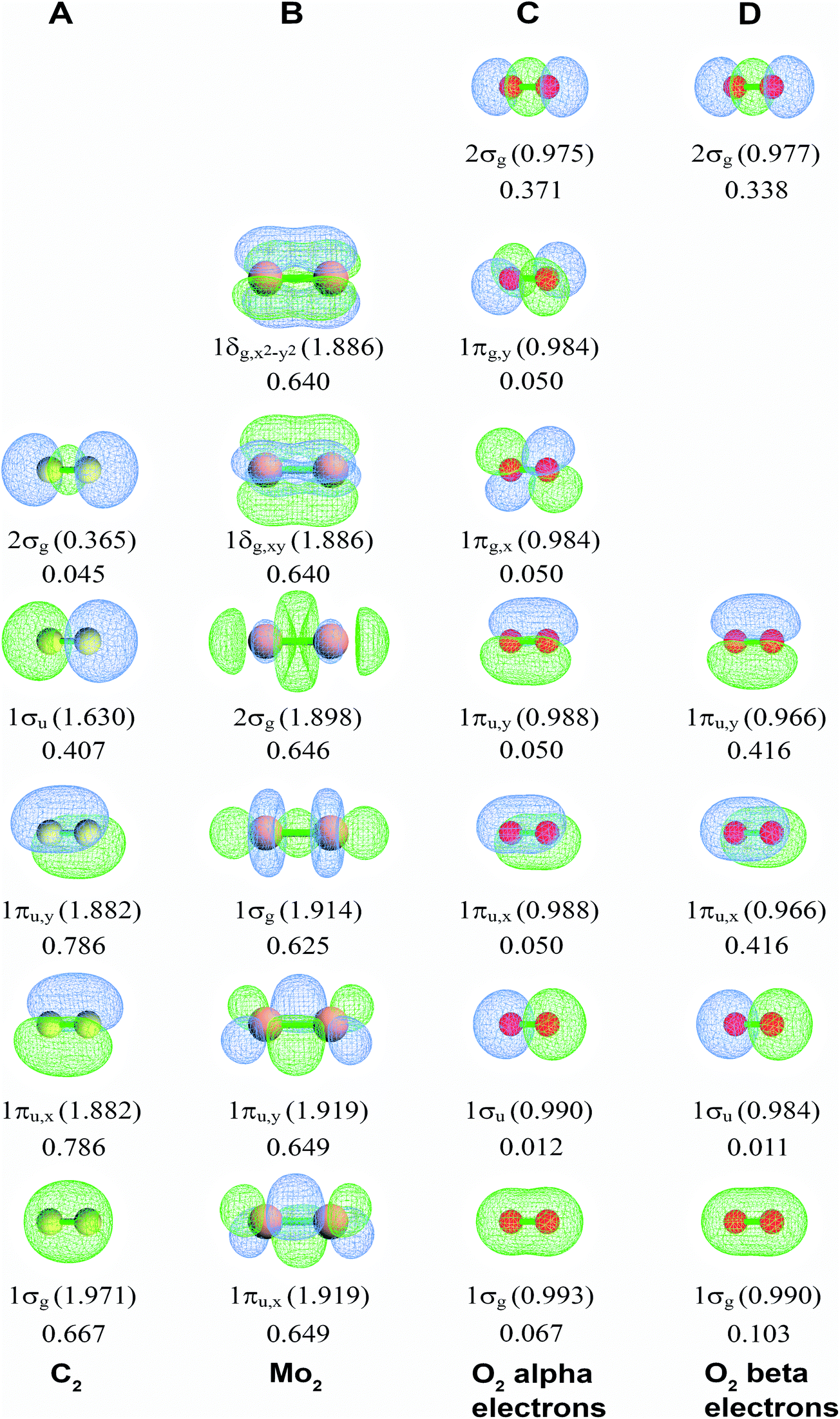
Bond Orders Of The Diatomic Molecules Rsc Advances Rsc Publishing Doi 10 1039 C9rad
Q Tbn 3aand9gcr01nkfsbchpzil6zzchbrv6vslsilt 8edhg0j6moe5oa6hqtz Usqp Cau

Atomic Orbital Wikipedia
.png)
How Do I Determine T Questioncove
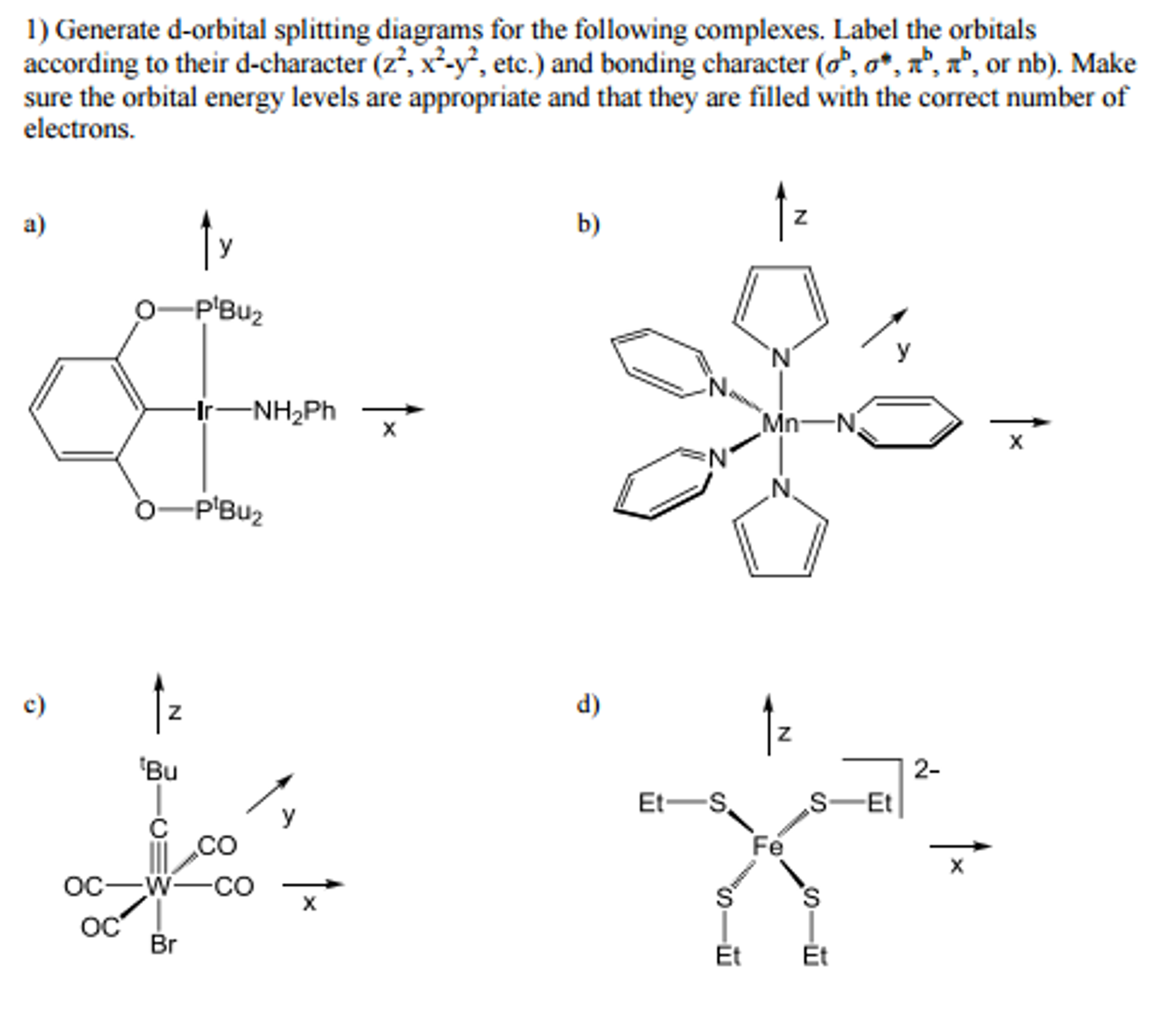
Generate D Orbital Splitting Diagrams For The Foll Chegg Com
Shapes Of The 3d Orbitals In 3d

Quantum Numbers A Short Tutorial Bohr Model Of Hydrogen Atom An E S Is Found In Specific Energy Levels These Levels Represent A Fixed Distance From Ppt Download
What Are The Nodal Planes Of The D X 2 Y 2 Orbital Socratic

Chapter 10 Coordination Chemistry Ii Bonding Pdf Free Download

How Are D Orbitals Named Dz Dxy Dyz Dxz And Dx Y Quora

Solved What Is The Symmetry Label For A D X 2 Y 2 Orbit Chegg Com

Shape Of The Antibonding T 2g Xy And E G X 2 Y 2 Orbitals Of Download Scientific Diagram

Visualizing Atomic Orbitals

Magnetic Quantum Number Chemistrygod

Is It 3dx2y2 Or 3dx2 Y2 Chemistry Structure Of Atom Meritnation Com

What Is Shape Of D Orbital Designated As Dxy Dyz Dxz Dx2 Y2 Dz2 Chemistry Structure Of Atom Meritnation Com

How Are D Orbitals Named Dz Dxy Dyz Dxz And Dx Y Quora

2 Chemistry Part 1 Pages 151 0 Flip Pdf Download Fliphtml5

What Is The Significance Of The 3d X 2 Y 2 Atomic Orbital Homeworklib
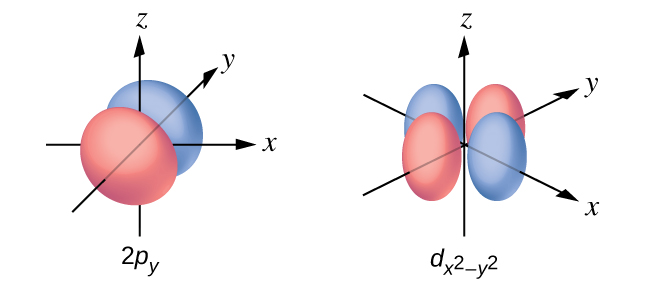
6 3 Development Of Quantum Theory Chemistry

Shapes Of Atomic Orbitals

Atomic Orbital Chemistrygod
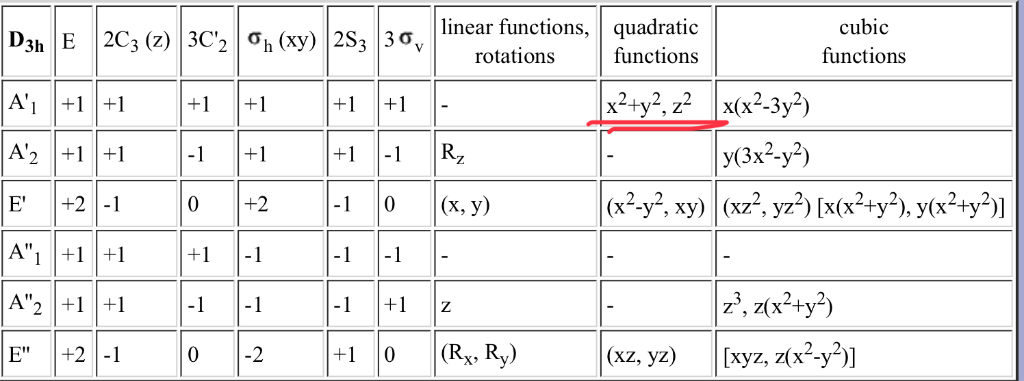
In The Character Table Which D Orbital Does X 2 Y Chegg Com
Spectroscopic And Magnetic Properties Course Hero

Draw The Shape Of Dz Orbital Brainly In

Interactive Student Tutorial

In Which Of The Following D X 2 Y 2 Orbital Is Not Participate In Its Hybridisation Youtube

6 6 3d Representation Of Orbitals Chemistry Libretexts

How Is The 5 Fold Degeneracy Of Atomic D Orbitals Removed In The Case Of Tetrahedral Compounds And Square Planar Compounds Study Com
Q Tbn 3aand9gcq2cre7gcfjri1ocpy A391bwszret5qevaztgqiy7cpnu8099b Usqp Cau
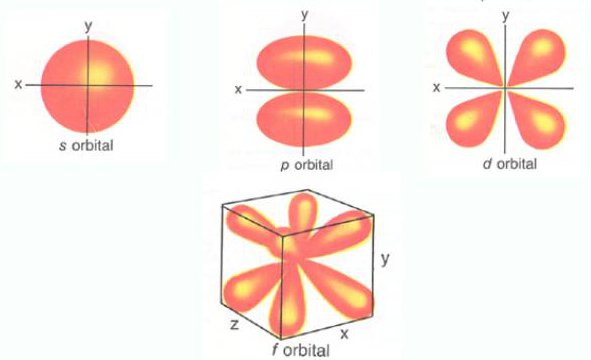
Orbit Subshell Orbital Electron

Quantum Number Wikipedia
Onlinelibrary Wiley Com Doi Pdf 10 1002 Ange
What Is The Difference Between Dx2 Y2and Dz2 Orbitals Quora

Hybrid Orbitals

Draw The Shape Of Dz Orbital Brainly In
Orbitals And Their Types S P D F Orbitals And Their Shapes
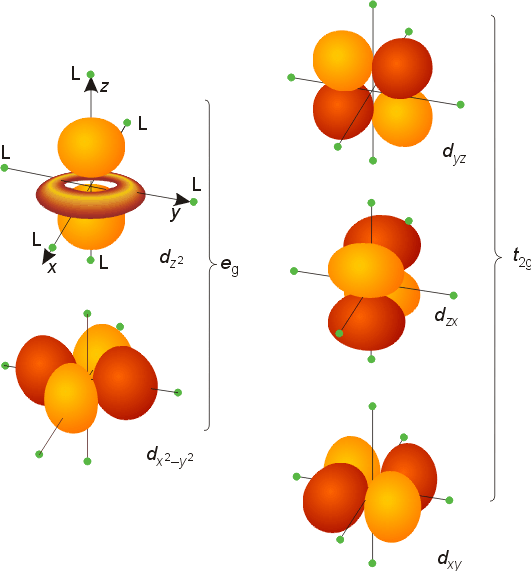
D Metal Complexes
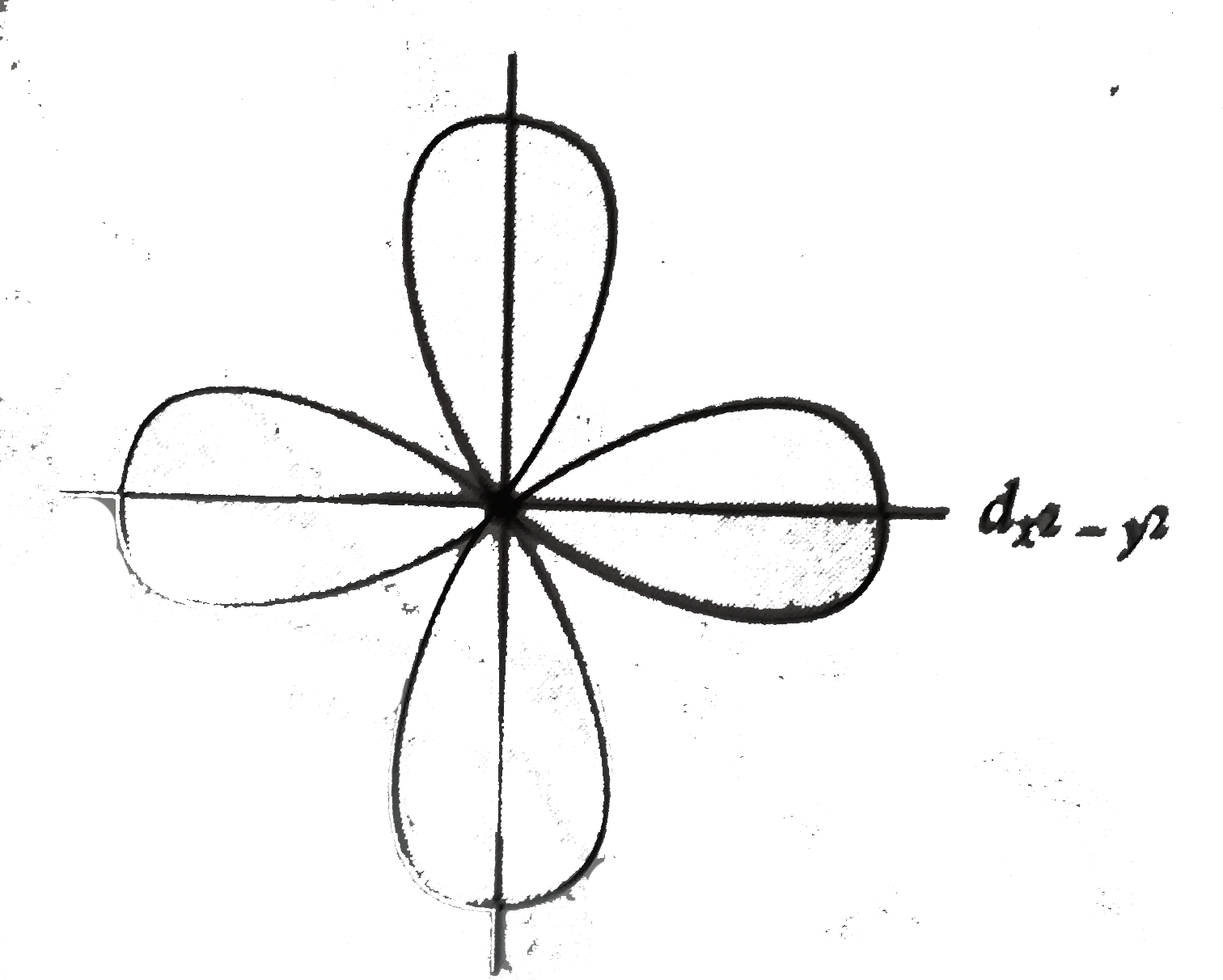
The Electron Density In The Xy Plane In 3d X 2 Y 2 Orbit

File Single Electron Orbitals Fz X2 Y2 Jpg Wikimedia Commons

Why How Do The Coefficients Associated With Atomic Orbitals Superposed To Form Hybrid Orbital Determine Their Spatial Orientation Physics Stack Exchange

What Is The Difference Between Dx2 Y2and Dz2 Orbitals Quora

Mn Eg Projected Dos In The Ground State A Afm In The X 2 Z 2 Y 2 Z Download Scientific Diagram
Complex Ions More About D Orbitals
Http Casey Brown Edu Chemistry Research Lswang Publications 360 Pdf
What Is The Significance Of The 3d X 2 Y 2 Atomic Orbital Socratic

A Copper D X 2 Y 2 Orbital And Its Surrounding Oxygen P X Or P Y Download Scientific Diagram
What Kinds Of Bonds Does The D X 2 Y 2 Orbital Form In Transition Metal Complexes Socratic

The Dx 2 Y 2 And Dz 2 Orbitals Are Directed Along A Set Of Mutually Perpendicular X Y And Z Axis And Are Called Eg Orbitals If True Enter 1 Else Enter 0

File Atomic Orbital Cloud N4 Fz X 2 Y 2 Png Wikimedia Commons

A The Five D Orbitals Dz2 Dx2y2 Dxy Dxy Dyz On The Central Transition Metal Of Complex Ions Not Used To Form Covalent Bonds To The Ligands Have The Same Energy Prior To

Within An Energy Level N 1 2 3 4 There Exists N Types Of Orbitals And N 2 Sublevels Norbital Types One S Orbital Three P Orbitals One S Orbital Ppt Download

What Is The Difference Between The D X 2 Y 2 And D Xy Orbitals Of The Same N Socratic

Schematic Diagram Of Cu 3 D X 2 Y 2 Orbitals And O 2 P S Download Scientific Diagram
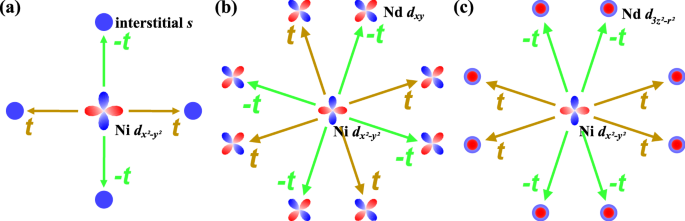
A Substantial Hybridization Between Correlated Ni D Orbital And Itinerant Electrons In Infinite Layer Nickelates Communications Physics
Complex Ions More About D Orbitals

Atomic Orbital Chemistrygod

Chemistry The Central Science Chapter 6 Section 6
Q Tbn 3aand9gctt Z3b6pn9vyt3gk4cgjc5ixxiwfobiceiv Cew Cv9kv6zmmi Usqp Cau
Shapes Of Dorbitals Assignment Help Homework Help Online Tutoring Chemistry Help
6 6 3d Representation Of Orbitals Chemistry Libretexts

File Fz X2 Y2 Orbital Png Wikimedia Commons
The Electron Density In The Xy Plane In 3d X 2 Y 2 Orbital Is

3dx2 Y2x Mov
Canvas Harvard Edu Courses 4068 Files Download Download Frd 1
Structure Reactivity Atoms
D X2y2 Orbital Superimosed On An Octahedral Model 3d Warehouse

The Roles Of 4f And 5f Orbitals In Bonding A Magnetochemical Crystal Field Density Functional Theory And Multi Reference Wavefunction Study Dalton Transactions Rsc Publishing Doi 10 1039 C6dte
Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry Atoms First Openstax Cnx

Molecular Nitrogen And Related Diatomic Molecules

Orbital Symmetry And Orbital Excitations In High T C Superconductors

Biochemistry Glossary Orbitals 2 Shape Draw It To Know It

Magnetic Quantum Number Chemistrygod

The Shape Of The 4d Orbital Chemistry X Youtube

Introduction To Orbitals Stahl 9 12
Opencommons Uconn Edu Cgi Viewcontent Cgi Article 1047 Context Chem Educ

Science Skool Atomic Orbitals

Geos 306 Fall 04 Lecture 2 The Nature Of The Atom

Molecular Orbital Theory Chemistry Openstax Cnx
Http W0 Rz Berlin Mpg De Imprs Cs Download Symmetry11 2 K Horn Pdf

Development Of Quantum Theory Chemistry

State True Or False The Electron Density In Xy Plane Of 3dx 2 Y 2 Orbital Is Zero




